The decision, made at a meeting on Thursday, comes amid a large increase in Covid cases throughout Europe, mainly attributed to low vaccination rates in some countries and to the fact that children under 11 years of age have so far not been given the vaccine.
In Spain, the highest 14-day cumulative incidence is among children eleven years old and younger. This is 203.57, much higher than the incidence for the next group, people between 40 and 49, which is 155.91.
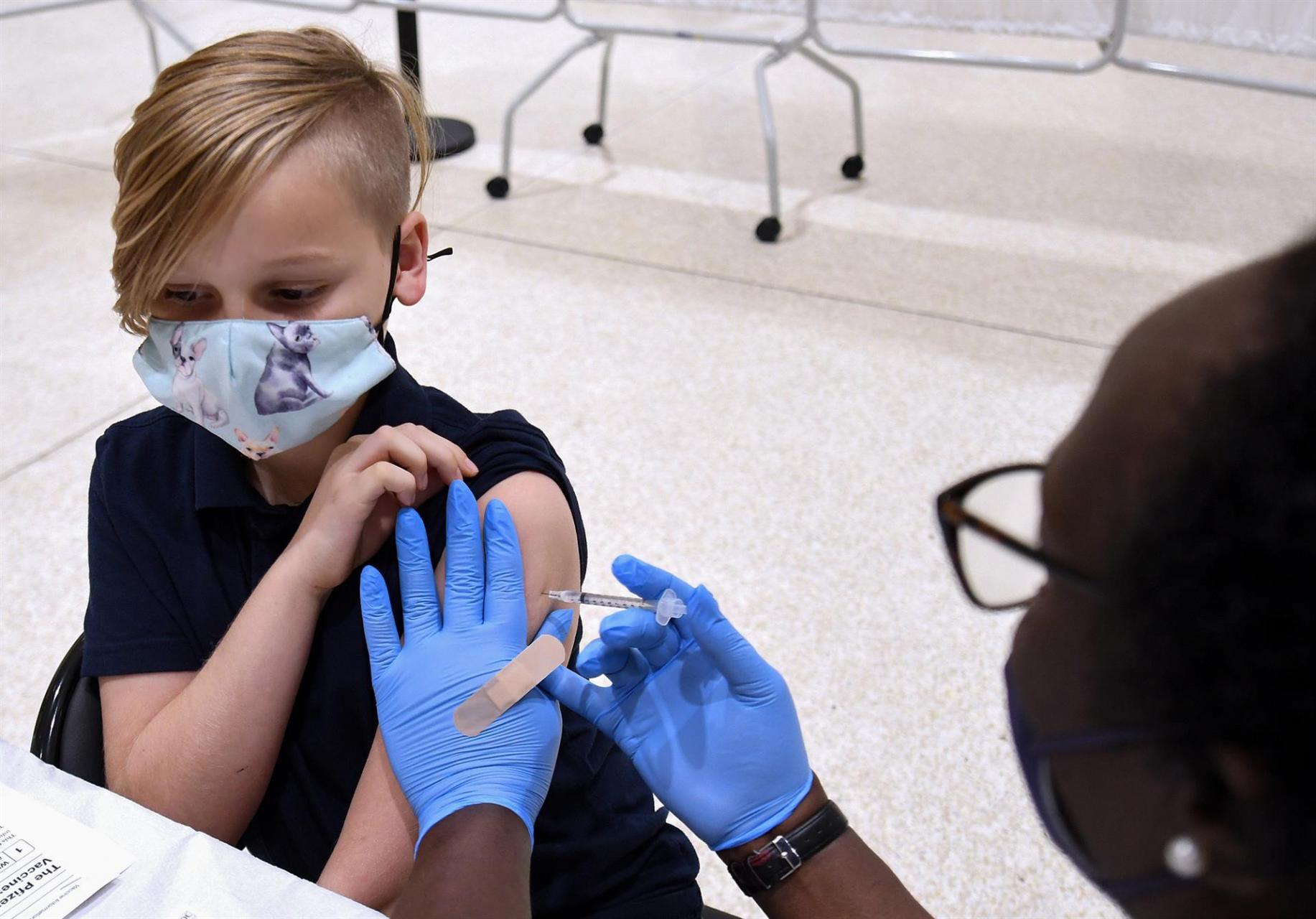
In the United States, Pfizer's vaccine has been licensed for children aged 5 to 11 for nearly a month. According to data from the US Food and Drug Administration, the vaccine was 90.7 per cent effective in preventing Covid-19 in this age group.
In addition, the safety of the vaccine was studied in approximately 3,100 children aged 5 to 11. No serious side effects were detected in a clinical trial that is still ongoing and which has now involved some 4,700 children. The study is being carried out in Spain, the United States, Finland and Poland.

No comments
To be able to write a comment, you have to be registered and logged in
Currently there are no comments.